
Cosmetic OEM and ODM
Strengths of KANAE TECHNOS
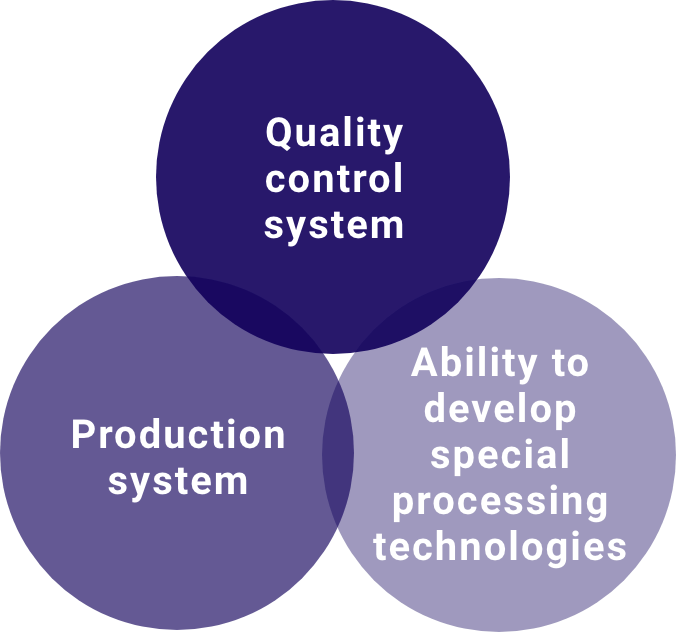
Leverage our specialized processing technologies, production systems, and development capabilities, quality control systems mainly to support manufacturing and sale of quasi-drugs, cosmetics, and miscellaneous goods using non-woven fabrics.
With our strengths in special processing technology, advanced production systems, and development capabilities, we respond quickly to diversifying customer and market needs.
In addition, we propose and develop new products
by leveraging many examples of approved quasi-drugs developed by the marketing authorization holder Elisent (a 100% subsidiary of KANAE TECHNOS).
Various marketing licenses
Quasi-drugs
Cosmetics
Pharmaceuticals
We can help you to obtain approval for a quasi-drug
At KANAE TECHNOS we obtain more than 15 quasi-drug approvals each year, including those obtained under the name of Elisent, our 100% subsidiary. We leverage these precedents to
help customers speed up the process of obtaining quasi-drug approvals.

Unique and special processing technologies cultivated over many years
Our departments of manufacturing, quality control, production technology, and development possess know-how and highly specialized technologies acquired through extensive achievements and experience. A great product is created by fusing specialized technologies.

We can flexibly
produce from small to large quantities
We effectively use machines capable of a wide range of processing for various specification requirements. We have built flexible production systems to meet user requirements regarding production volume and delivery time.

Development capabilities in pursuit of functionality and stability
At the product proposal and development stage, we conduct the same stability tests (aging tests) required for pharmaceuticals, not only on the physical properties of products but also on packaging materials and non-woven fabrics.
We conduct rigorous product development considering not only the distribution stage after production but also the stage of delivery to and use by consumers.

About Quality Control
KANAE TECHNOS, as a quality-oriented group of manufacturing professionals,
ensures thorough quality control and delivers safe and secure products.
Establish high-quality production systems based on good manufacturing practice (GMP)
The Head Office Factory, Head Office No. 2 Factory, and Kanonji Factory have acquired ISO 22716 certification, or GMP for cosmetics, and our high-quality production systems have earned a good reputation among our customers.
- Subjects: Head Office Factory, Head Office Factory No. 2, and Kanonji Factory

- – We have achieved consolidated production by procuring raw materials, preparing chemicals, packaging products, applying stickers, and packing products in boxes.
- – We have basically automated steps from cutting (clipping), to folding, impregnating, and packaging non-woven fabric. In addition, the possibility of contamination with foreign substances and microorganisms is minimized by adopting one-way routes for the flow of people and logistics.
- – Most of our production facilities use SUS316 and SUS316L parts, and our products are manufactured in Class 100,000 cleanrooms.
- – Periodic monitoring of airborne bacteria and airborne particulates ensures that the environment required for Class 100,000 clean rooms is maintained.
Introduction of advanced sanitation control systems and sterilization equipment

De-Ionized (DI) Water System
For production water (process water), a unique hydrothermal circulation system “DI (De-Ionized) Water System” is used. Purified water conforming to the Japanese Pharmacopoeia is thermally circulated and cooled to ensure use of microbial-free water.

EOG Sterilization Equipment
This equipment uses ethylene oxide gas (EOG) to sterilize objects. This allows you to control the bioburden of non-woven fabrics and increase the range of non-woven fabrics and chemicals that can be combined.
OEM Flow
FLOW01
Meeting
We will listen to your needs and suggest products that meet them.
FLOW02
Trial production, selection of specifications, selection of non-woven fabric
Formulation and non-woven fabric specifications will be examined and prototypes will be made.
FLOW03
Determine specification and estimate
Even at the prototyping stage, if the specifications are determined we can develop a rough estimate.
FLOW04
Formulation, final selection of non-woven fabric, and final estimate
We make a final estimate
when the specifications of the formula and non-woven fabric are determined.
FLOW05
Test
We will conduct a stability test
and a preservative efficacy test (challenge test) for the determined formulation and non-woven fabric specifications.
- Stability test:
If the product is stable at 40°C and 75% humidity for six months, we can guarantee the product quality at room temperature for three years. - Preservative efficacy test
(antimicrobial activity test: challenge test):
In accordance with the Japanese Pharmacopoeia, the test is conducted by adding a certain number of bacteria to the product and confirming the decrease in the bacteria count to evaluate safety against bacterial contamination.
- Stability endpoints:
- Appearance (color), pH, odor, (quantitative value of indicator substance, if necessary)
seal strength, and laminate strength
- Evaluation criteria and duration:
- 40°C/75% humidity for one month, three months, and six months (5°C as a control)
50°C (spontaneous humidity) for one month
- – Items, conditions, and periods will be discussed with the customer in advance. Results are periodically reported.
FLOW06
Manufacturing and delivery
We will manufacture and deliver the quantity you ordered under strict quality control.
FLOW07
Follow-up
We will not only accept repeat orders but also propose renewal plans and new products.
Product Range

Skin care sheets
In addition to highly functional face masks and gel sheets using proprietary technologies, we also develop and provide products with new textures, such as cream masks and melting sheets.
Skin care sheets
Wet sheets
We supply wet sheets that can be used for pharmaceuticals, quasi-drugs, cosmetics, toiletries, automotive products, and many other applications.

Chemical filling
We can also fill chemicals into sachets, pouches, bottles, etc. We have experience in filling a variety of products from skin care to hair care products.
Chemical filling